
Beretta SO4 Photo Click to Enlarge Artisan Stock and Gunworks
Sulfate is a very weak oxidizing agent. Since sulfur is in its maximum oxidation number in sulfate ion, this ion cannot act as a reducing agent. This page titled Sulfate Ion (SO₄²⁻) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by James P. Birk. Sulfate ion is a very weak base.

Natri Sulfate (AR) (NH4)2Fe(SO4)2.6H2O Vật Tư Bách Khoa
Ammonium iron(II) sulfate hexahydrate is a salt used in various applications, such as analytical chemistry, biochemistry, and dyeing. It is also known as Mohr's salt and has the formula (NH4)2Fe(SO4)2 · 6H2O. Learn more about its properties, synthesis, and uses at MilliporeSigma, a leading supplier of chemical and biological products.

Beretta SO4 Photo Click to Enlarge Artisan Stock and Gunworks
Word Equation. Aluminium + Sulfuric Acid = Aluminum Sulfate + Dihydrogen. Al + H2SO4 = Al2 (SO4)3 + H2 is a Single Displacement (Substitution) reaction where two moles of solid Aluminium [Al] and three moles of aqueous Sulfuric Acid [H 2 SO 4] react to form one mole of aqueous Aluminum Sulfate [Al 2 (SO 4) 3] and three moles of Dihydrogen [H 2.

Beretta SO4 Sporting Shotgun
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula Fe SO 4 ·xH 2 O. These compounds exist most commonly as the heptahydrate (x = 7) but several values for x are known.The hydrated form is used medically to treat or prevent iron deficiency, and also for industrial applications.Known since ancient times as copperas and as green.

今日のメモBeretta SO4
Sodium chloride is an ionic compound composed of sodium cations, Na +, and chloride anions, Cl −, combined in a 1:1 ratio. The formula mass for this compound is computed as 58.44 amu (see Figure 3.4 ). Figure 3.4 Table salt, NaCl, contains an array of sodium and chloride ions combined in a 1:1 ratio. Its formula mass is 58.44 amu.

BERETTA SO4
The eastern United States was a prominent example: the temperatures there declined by 0.7 °C (1.3 °F) between 1970 and 1980, and by up to 1 °C (1.8 °F) in the Arkansas and Missouri. As the sulfate pollution was reduced, the central and eastern United States had experienced warming of 0.3 °C (0.54 °F) between 1980 and 2010, [30] even as.

Aluminium Sulfate [Al2(SO4)3] 99 AR Grade Powder 4 Oz in a Plastic Bottle USA eBay
Mnemonic. The letters represent the extraocular muscles and numbers represent their respective cranial nerve supply: LR6: lateral rectus, innervated by the 6 th (abducens) nerve. SO4: superior oblique, innervated by the 4 th (trochlear) nerve. O3: other muscles ( superior, inferior, medial recti and inferior oblique ), innervated by the 3 rd.

あるたろぉ ̗̀ 𖤐 (ar_so4) / Twitter
Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na 2 SO 4 as well as several related hydrates.All forms are white solids that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product. It is mainly used as a filler in the manufacture of powdered home laundry.

Beretta SO4
Sulfur has 6 valence electrons. Each oxygen atom has 6 valence electrons. Since sulfate has 4 oxygen atoms, that equals 24 valence electrons. Sulfate has a charge of 2 −, which means it has an.

Beretta SO4 for sale at 987519443
3. Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in Na2SO4: Molar Mass (g/mol) Na (Sodium) 2 × 22.98976928 = 45.97953856. S (Sulphur/Sulfur) 1 × 32.065 = 32.065. O (Oxygen)

Buy Aluminium Sule [Al2(SO4)3] 99 AR Grade Powder 1 Lb in Two SpaceSaver Bottles USA Online at
Molar mass of SO4 is 96.0626 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between SO4 weight and moles. Compound.

Aluminium Sulfate [Al2(SO4)3] 99 AR Grade Powder 8 Oz in a Bottle USA Garden & Outdoor
Formula in Hill system is ArO12S3: Computing molar mass (molar weight) To calculate molar mass of a chemical compound enter its formula and click 'Compute'.

あるたろぉ ̗̀ 𖤐 (ar_so4) / Twitter
Formula in Hill system is Ar O 4 S: Computing molar mass (molar weight) To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Capitalize the first letter in chemical symbol and use lower case for the remaining letters: Ca, Fe, Mg, Mn, S, O, H, C, N, Na, K, Cl.

Beretta SO4 Coombe Farm
The importance of tissue sulfate concentrations in regulating 3'-phosphoadenosine 5'-phosphosulfate (PAPS) synthesis is not known. Therefore, this study was conducted to determine the influence of increased availability of inorganic sulfate on steady-state PAPS concentrations in various tissues. To increase tissue sulfate concentrations, 2-16 mmol/kg of sodium sulfate and sulfur-containing.
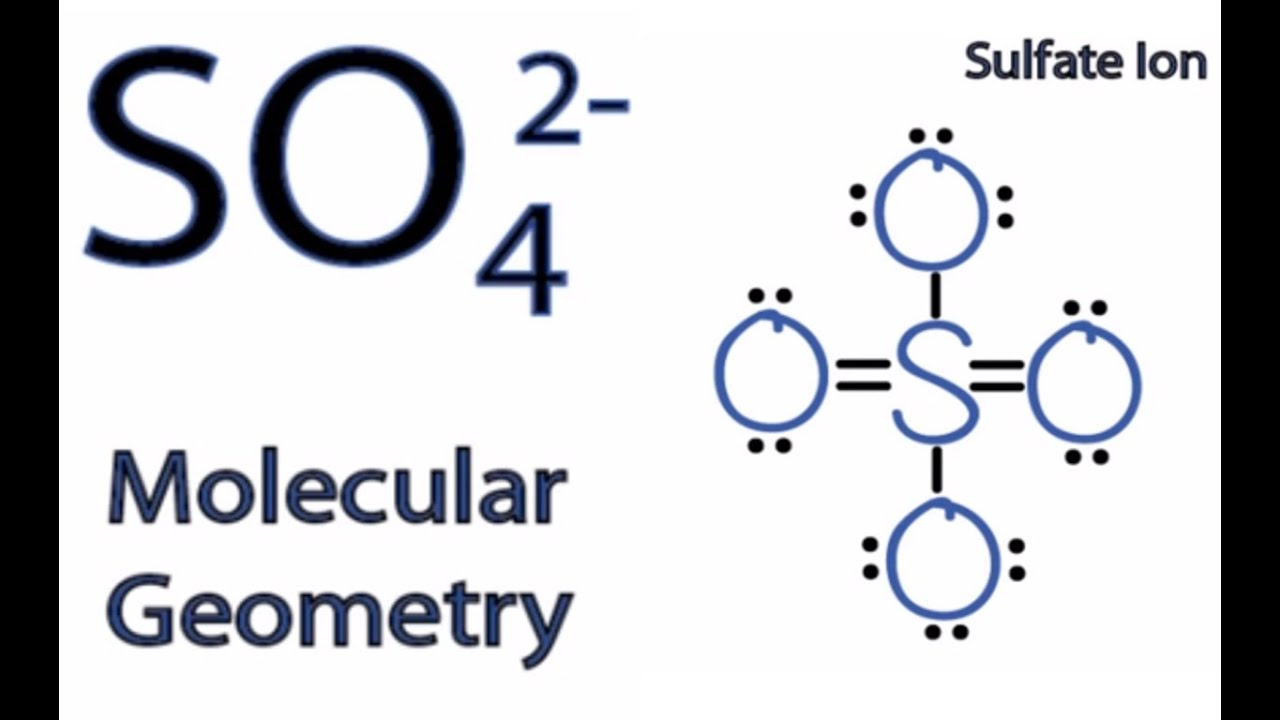
Lewis Structure Of Sulfate Ion
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.

Beretta SO4 Sporting 12ga for sale
Magnesium sulfate or magnesium sulphate is a chemical compound, a salt with the formula MgSO 4, consisting of magnesium cations Mg 2+ (20.19% by mass) and sulfate anions SO 2− 4.It is a white crystalline solid, soluble in water but not in ethanol.. Magnesium sulfate is usually encountered in the form of a hydrate MgSO 4 ·nH 2 O, for various values of n between 1 and 11.